how to calculate density g cm3 Density of materials lesson 1.2
In this post, we will explore the concept of density and how it relates to two different scenarios involving a diamond and a solid cube. Density is a fundamental property of matter and is often used to describe how tightly packed or compact a substance is. It is defined as the mass of an object divided by its volume. Let’s delve into these examples to better understand density.
A Diamond’s Density
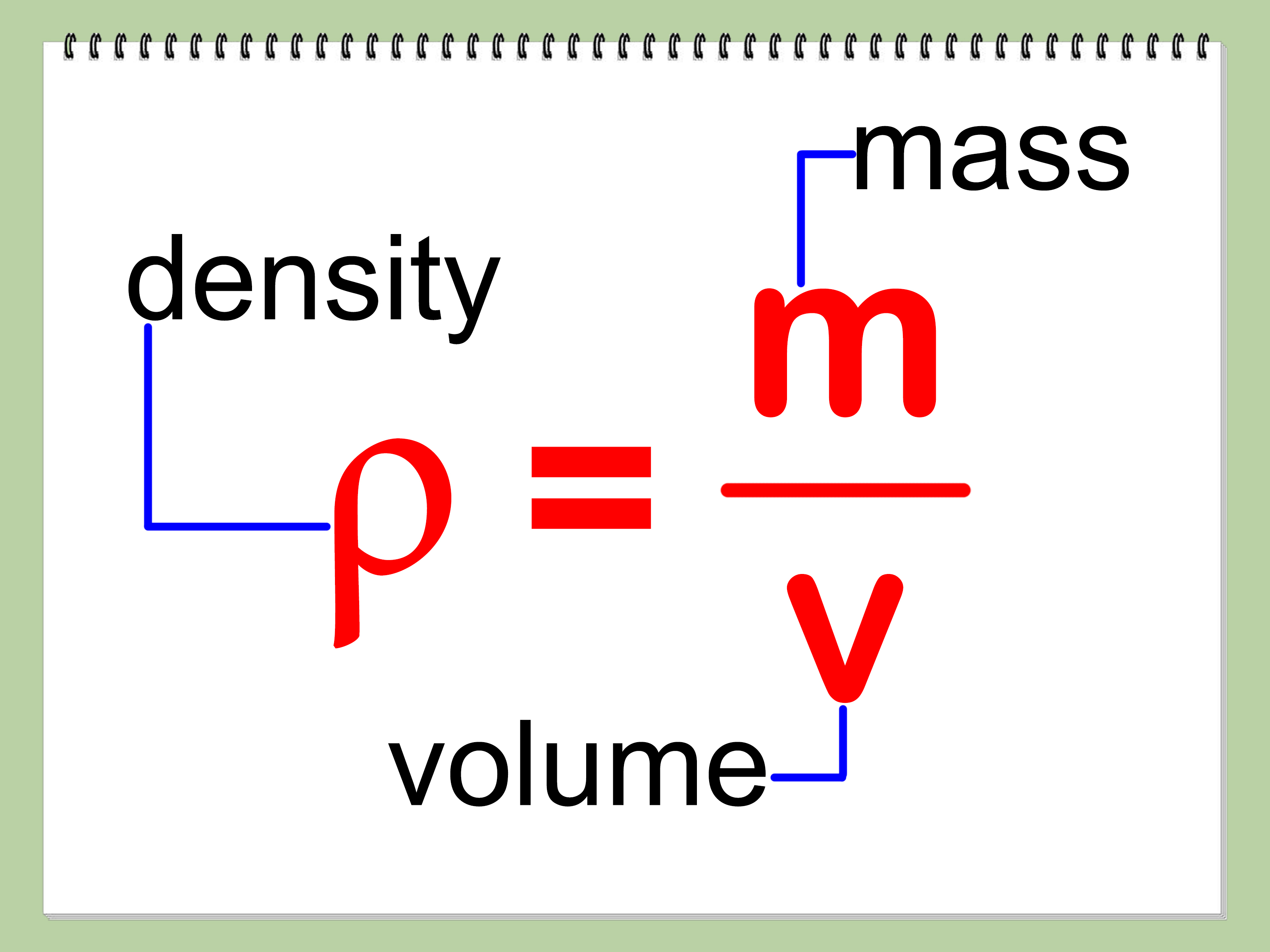 Have you ever wondered how we can determine the density of a diamond? Well, let’s consider a diamond with a mass of 1.76 grams and a volume of 0.5 cubic centimeters. To find the density, we simply divide the mass by the volume. In this case, the density of the diamond can be calculated as:
Have you ever wondered how we can determine the density of a diamond? Well, let’s consider a diamond with a mass of 1.76 grams and a volume of 0.5 cubic centimeters. To find the density, we simply divide the mass by the volume. In this case, the density of the diamond can be calculated as:
Density = Mass / Volume
Density = 1.76 g / 0.5 cm3
After performing the calculation, we find that the density of the diamond is 3.52 grams per cubic centimeter (g/cm3). This means that for every cubic centimeter of the diamond, it has a mass of 3.52 grams.
The Density of a Solid Cube
 Now, let’s shift our focus to a solid cube. Imagine we have a cube with an unknown mass and volume. To determine its density, we need both pieces of information. Thankfully, our trusty equation, Density = Mass / Volume, can help us out once again!
Now, let’s shift our focus to a solid cube. Imagine we have a cube with an unknown mass and volume. To determine its density, we need both pieces of information. Thankfully, our trusty equation, Density = Mass / Volume, can help us out once again!
Suppose the mass of the solid cube is 120 grams. If the edge length of the cube is 5 centimeters, the volume of the cube can be calculated as follows:
Volume = Edge Length x Edge Length x Edge Length
Volume = 5 cm x 5 cm x 5 cm
After performing the calculation, we find that the volume of the cube is 125 cubic centimeters. Now, we can determine the density:
Density = Mass / Volume
Density = 120 g / 125 cm3
By dividing the mass by the volume, we find that the density of the solid cube is 0.96 grams per cubic centimeter (g/cm3). This implies that for every cubic centimeter of the cube, it has a mass of 0.96 grams.
Understanding the concept of density is crucial in various scientific and real-life situations. It allows us to compare the compactness of different substances and helps in identifying materials or determining their composition based on their density. By applying the simple formula of Density = Mass / Volume, we can unlock a wealth of information about the physical properties of different objects. Remember to divide the mass by the volume to find the density!
If you are looking for Solved What is the density, in g/cm3,of a solid cube that is | Chegg.com you’ve visit to the right page. We have 5 Pictures about Solved What is the density, in g/cm3,of a solid cube that is | Chegg.com like Solved What is the density, in g/cm3,of a solid cube that is | Chegg.com, Solved Calculate the density, in g/cm3 , of a metal that has | Chegg.com and also Solved What is the density, in g/cm3,of a solid cube that is | Chegg.com. Here you go:
Solved What Is The Density, In G/cm3,of A Solid Cube That Is | Chegg.com
 www.chegg.comcm3 density cube solid feet transcribed text solved show
www.chegg.comcm3 density cube solid feet transcribed text solved show
The Mass Of A Diamond Is 1.76 G. The Volume Is 0.5 Cm^3. What Is The
 socratic.orgvolume massa rumus calculate unit densita fisika tabel contoh molecules calcolare
socratic.orgvolume massa rumus calculate unit densita fisika tabel contoh molecules calcolare
Solved Calculate The Density, In G/cm3 , Of A Metal That Has | Chegg.com
 www.chegg.comcalculate density cm3 metal solved show
www.chegg.comcalculate density cm3 metal solved show
Density Of Materials Lesson 1.2 - ACA Grade 8 Science
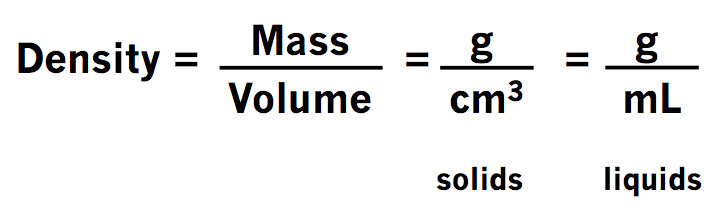 acamrmicheal.weebly.comdensity volume ml unit mass formula equation measuring divided kg lesson solids assignment summer densities liquids grams science grade answer
acamrmicheal.weebly.comdensity volume ml unit mass formula equation measuring divided kg lesson solids assignment summer densities liquids grams science grade answer
PPT - Measurement/Calculation PowerPoint Presentation, Free Download
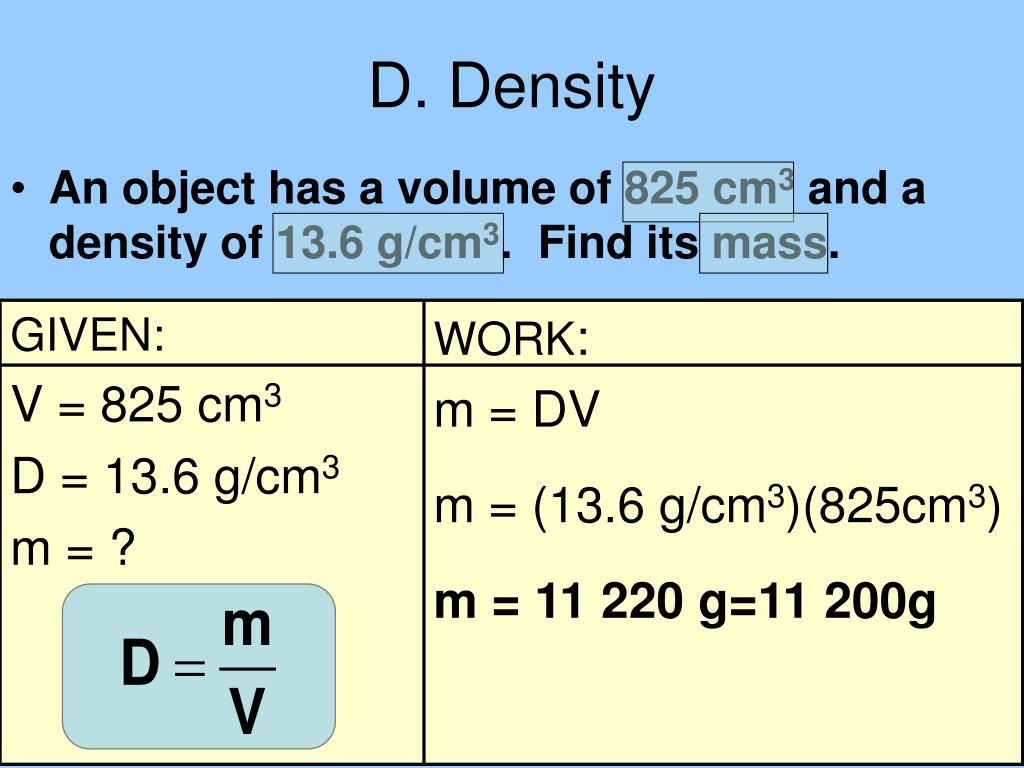 www.slideserve.comdensity measurement calculation cm3 volume ml unit derived ppt powerpoint presentation mass find
www.slideserve.comdensity measurement calculation cm3 volume ml unit derived ppt powerpoint presentation mass find
The mass of a diamond is 1.76 g. the volume is 0.5 cm^3. what is the. Volume massa rumus calculate unit densita fisika tabel contoh molecules calcolare. Density measurement calculation cm3 volume ml unit derived ppt powerpoint presentation mass find